|
強(qiáng)生(JNJ)丙肝新藥OLYSIO(simeprevir)獲歐盟批準(zhǔn),聯(lián)合其他藥物,用于丙型肝炎病毒(HCV)基因型1和4慢性丙型肝炎(CHC)成人患者的治療。該藥將為歐洲患者提供一種新的三聯(lián)療法,同時將提供有史以來首個為期12周的無干擾素且不依賴?yán)晚f林及其他藥物的治療選擇。
OLYSIO的獲批,是基于II期COSMOS、3個關(guān)鍵III期QUEST-1、QUEST-2、PROMISE的數(shù)據(jù)。丙型肝炎(HCV)是一種血源性傳染性肝臟疾病,若不及時治療,可能對肝臟造成重大損害。
關(guān)于OLYSIO(simeprevir):
Simeprevir是新一代NS3/4A蛋白酶抑制劑,為每日一次的口服藥物,由Medivir公司和楊森(Janssen)聯(lián)合開發(fā),用于治療慢性丙型肝炎成年患者的代償性肝病,包括各個階段的肝纖維化,其工作原理是通過阻斷蛋白酶,來抑制HCV在肝臟細(xì)胞中的復(fù)制。
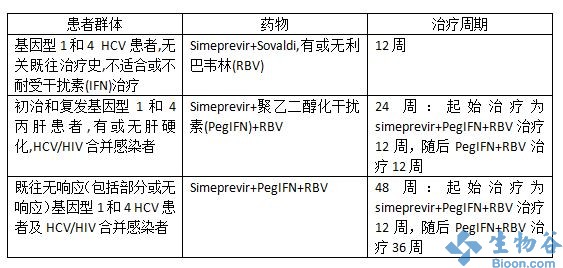
simeprevir分別于2013年9月和11月獲日本(在日本的商品名為Sovriad)和FDA批準(zhǔn),與聚乙二醇化干擾素和利巴韋林(ribavirin)聯(lián)合用藥,用于基因型-1慢性丙型肝炎病毒(HCV)感染者的治療。
simeprevir是一種新的直接作用抗病毒藥物,也是第二代蛋白酶抑制劑,給藥方式為:simeprevir+聚乙二醇干擾素+利巴韋林聯(lián)合治療12周,隨后進(jìn)行聚乙二醇干擾素+利巴韋林治療12周或36周。
閱讀摘要原文
OLYSIO™ (simeprevir) receives marketing authorisation in the European union for the treatment of adults with hepatitis C genotype 1 and 4 infection
Simeprevir provides a new triple therapy treatment option, as well as the first ever 12-week interferon-free and ribavirin independent treatment regimen, in combination with sofosbuvir, for appropriate patients in Europe
BEERSE, BELGIUM [May 16, 2014] Janssen-Cilag International NV today announced that its next generation protease inhibitor (PI) OLYSIOTM (simeprevir) has been granted marketing authorisation by the European Commission (EC) for the treatment of adults with genotype 1 and 4 chronic hepatitis C (CHC), in combination with other medicinal products, which includes1:
This marketing authorisation represents a significant milestone in the development of new triple therapy hepatitis C (HCV) treatment options for genotype 1 and 4 patients. It also includes simeprevir as part of an all oral 12-week IFN-free direct-acting antiviral (DAA) regimen with or without RBV, in genotype 1 or 4 patients, who are intolerant to or ineligible for IFN treatment.[1]
“The EC marketing authorisation for simeprevir is a great milestone as it adds an important new treatment option for patients, demonstrating the continued role of triple therapy in the treatment of HCV. In addition, the introduction of an all oral, 12-week interferon-free treatment regimen provides a new option for sustained virologic response in HCV patients with genotypes 1 or 4 intolerant to or ineligible for interferon-based treatment,” said Thomas Stark, Medical Director, Janssen EMEA.
HCV represents a major global public health concern. There are an estimated nine million people[2] living with HCV in Europe which, if untreated, can cause severe damage to the liver, including cirrhosis and hepatocellular carcinoma (HCC). HCV represents a leading cause of liver transplantation in Europe.[3] Whilst the number of patients being newly diagnosed with HCV is declining, it takes approximately 20 – 30 years for symptoms to appear, with HCV cases expecting to peak between 2030 and 2035.[4],[5]
Dr Andrew Ustianowski, Chair of the British Viral Hepatitis Group and Consultant in Infectious Diseases at North Manchester General Hospital, commented: “The treatment environment in hepatitis C infection is evolving rapidly. Simeprevir is a well-tolerated and efficacious addition to our therapies against hepatitis C, and is a very welcome development for both those with genotype 1 and those with genotype 4.”
The EC marketing authorisation for simeprevir with PegIFN + RBV is based on a clinical trial programme involving three pivotal Phase 3 studies, with over 1000 patients. The trials, QUEST-1, QUEST-2[6] and PROMISE[7], explored the use of simeprevir in combination with PegIFN + RBV in treatment-naïve patients and patients who have relapsed after prior interferon-based treatment. All three studies met their primary endpoints and demonstrated that simeprevir, in combination with PegIFN + RBV, achieves significant sustained virological response rates when compared with PegIFN + RBV alone.
The EC marketing authorisation for the combination of simeprevir and sofosbuvir also contains results from the Phase 2 study, COSMOS, in treatment-naïve patients. This was based upon prior null responder and treatment-naïve patients.[8]
Simeprevir is taken once-daily for 12 weeks, with treatment-naïve and prior-relapser patients receiving pegylated interferon and ribavirin for 24 weeks, and for 48 weeks total by those shown to be prior non-responder patients (including partial and null responders)1. It is generally well tolerated, with the most common adverse events reported in clinical trials (incidence ≥ 5%) including nausea, rash, pruritus, dyspnoea, blood bilirubin increase and photosensitivity reaction.1
In March 2013, simeprevir was approved for the treatment of genotype 1 HCV in Japan, in Canada in September 2013, and the U.S. in November 2013, with the most recent approval occurring in Russia in March 2014. Following the EC marketing authorisation, it is anticipated that simeprevir will be available across a number of European union countries, in conjunction with reimbursement, in the second half of 2014.
About Simeprevir
Simeprevir is an NS3/4A protease inhibitor jointly developed by Janssen R&D Ireland and Medivir AB.
Janssen is responsible for the global clinical development of simeprevir and has exclusive, worldwide marketing rights, except in the Nordic countries. Medivir AB retains marketing rights for simeprevir in these countries under the marketing authorisation held by Janssen-Cilag International NV. Simeprevir was approved for the treatment of genotype 1 hepatitis C in September 2013 in Japan, in November 2013 in Canada and the U.S., and in March 2014 in Russia.
|